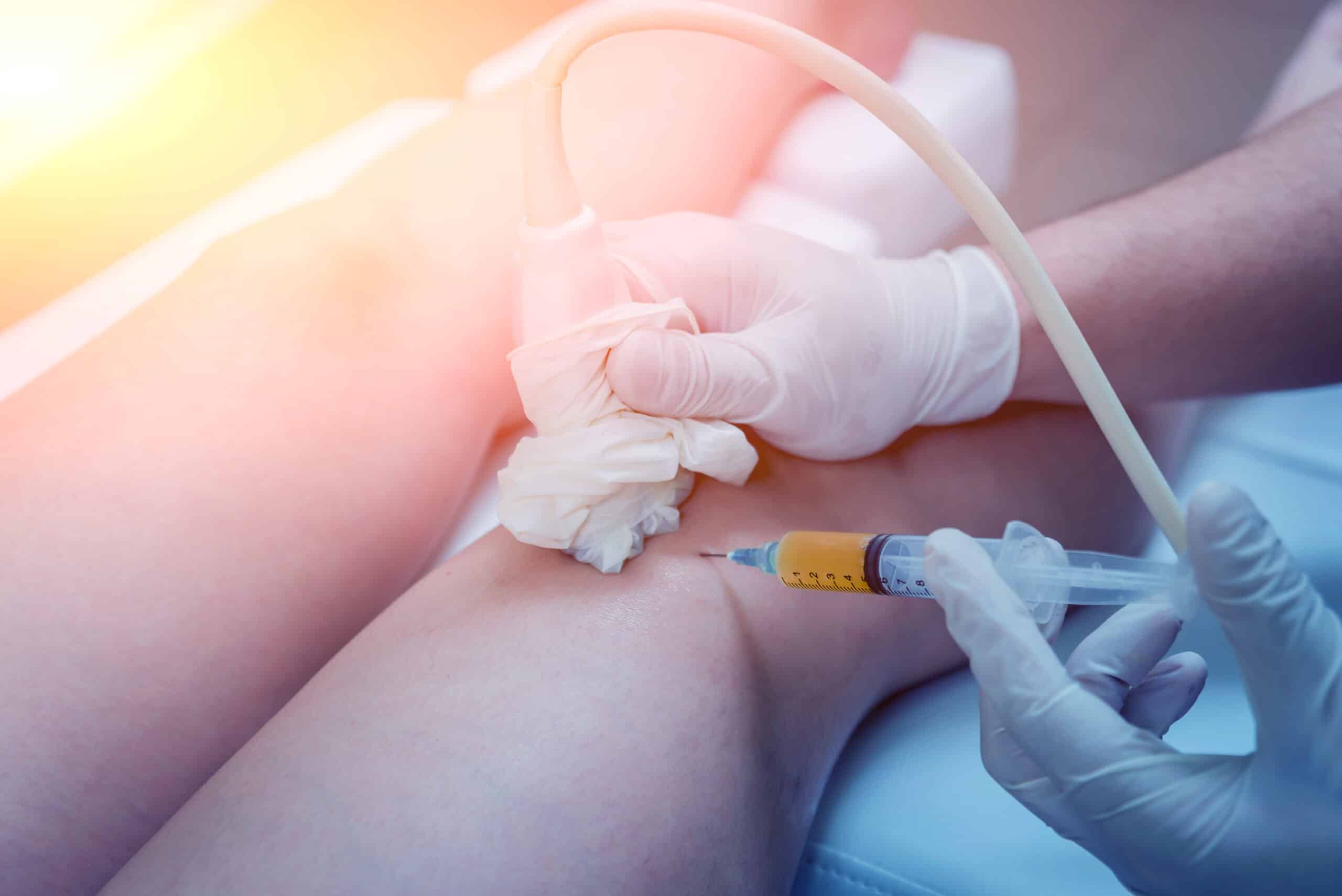
The results, published in the journal Regenerative Medicine, showed that a single injection of leukocyte-rich/PRP in the knee joint significantly improved functional mobility, pain and quality of life after six weeks. The study supports using this combined approach to further evaluate this and other emerging biological therapies for musculoskeletal disorders in larger clinical trials.
“OA is a leading cause of disabilities, affecting nearly 52 million Americans,” said first and corresponding author Dr. Prathap Jayaram, director of regenerative sports medicine and assistant professor in the Department of Physical Medicine and Rehabilitation and Orthopedic Surgery at Baylor. “It has been estimated that more than 80% of individuals older than 55 years have some X-ray-based evidence of the disease.”
OA develops when the smooth cushion between bones, the cartilage, breaks down. Progressively, joints become painful, swollen and hard to move, Jayaram explained. Currently, there are no validated therapies that delay disease progression. The current standard of care is limited to the alleviation of symptoms with corticosteroids.
“However, although steroids seem to be helpful in the short term for pain, emerging evidence has associated steroid long-term use in OA with loss of cartilage,” Jayaram said. “As OA is a whole joint disease, there is a need for developing novel therapeutic strategies that ultimately prevent and/or delay disease progression while improving functional outcomes. PRP is emerging as one of the promising candidates to treat OA that are currently being used in clinical practice.”
Combining wearable technology and patient assessment to evaluate the treatment
One challenge of previous studies assessing PRP therapies in OA is that treatment evaluation is based on patient-reported outcomes that subjectively assess pain or aspects of joint function, such as the time up-and-go (TUG), how quick a person gets up from a chair.
In this study, Jayaram and colleagues incorporated wearable technology to objectively assess functional outcomes such as TUG, in addition to patient-reported outcomes to comprehensively evaluate the efficacy of PRP in knee OA (KOA).
The prospective pilot study included 12 patients diagnosed with KOA. Each patient received one ultrasound-guided injection of PRP and function and pain were evaluate six weeks later.
PRP is prepared from the patient’s own blood by removing the red blood cells and enriching the concentration of platelets. PRP also contains white blood cells, or leukocytes. PRPs are formulated either leucocyte-rich (LR) or leukocyte-poor. A preclinical study by Jayaram and his colleagues had previously shown that LR-PRP had potential disease-modifying effects that correlated with functional outcomes.
“In the current study, we found that a single injection of LR-PRP into the knee does significantly improve functional mobility, pain and quality of life at six weeks,” Jayaram said. “To our knowledge, our study is the first to report the efficacy of LR-PRP on objective functional outcomes using wearable sensor technology and validated patient-reported outcomes. Our findings provide the basis to conduct larger randomized clinical trials of PRP.”
This research was funded by the National Heart, Lung and Blood Institute (5T32HL139430-02), the National Institute on Aging (1R42AG060853-01), the HAE AHM Sea Rock Foundation and the Charlotte and Jamil Azzam Foundation. Further support was provided by NIH grants (1R42AG060853-01 and 5T32HL139430-02).