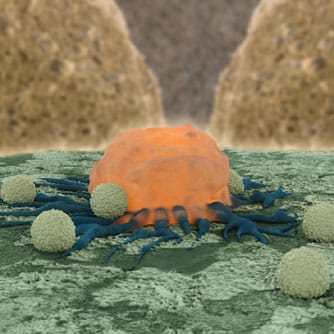
Today, many patients with newly diagnosed blood disorders – ranging from cancer to rare genetic conditions – respond well to modern treatment regimens. However, for more than half of newly treated patients, therapies fail to work or patients experience a relapse that may negatively affect their prognosis. Scientists are devising precision techniques to attack the specific targets that are responsible for a patient’s disease. Using a patient’s own re-engineered cells to attack their disease is an example of this approach. Building on the existing concept of turning the immune system into a disease-fighting weapon, this new field of medicine adds innovative technologies that transform healthy cells into “super” cells that can more effectively combat disease. One such method is chimeric antigen receptor (CAR) cell engineering. The CAR process starts when T cells (naturally occurring immune cells) are extracted from the blood of an individual and outfitted with two powerful features: a receptor on the outer cell surface that recognizes a protein called CD19 present on most leukemic cells and a powerful mechanism inside the cell that triggers it to expand and proliferate once attached to the targeted protein. With these new engineered features, the T cells are injected back into the patient, now primed to seek and destroy cancer cells. Studies on the CAR approach provide data on both adult and pediatric patients with leukemia who have responded well to this treatment strategy. Preliminary studies have found that this process may generate responses in as many as two-thirds of cases in which all other treatment options have failed. Further, because the cells are derived from the patient, there is an inherently lower risk of toxicity because the cells are less likely to attack the host tissue than cells introduced from a foreign body.
High-Tech Gene Therapy for Blood Disorders
Porter D. “Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019 cells) Have Long-Term Persistence and Induce Durable Responses In Relapsed, Refractory CLL” [Abstract 4162]. Presented at 55th American Society of Hematology Annual Meeting, December 9, 2013.
RELATED ARTICLES