The US DNA sequencing company Illumina started noticing something odd about 7 years ago, in testing involving 125,000 pregnant women looking for genetic abnormalities in their foetuses which yielded very unexpected signals in 10 cases. However the abnormal DNA they found wasn’t from the foetuses, rather it was undiagnosed cancer in the women. Alex Aravanis said “the test wasn’t developed for cancer screening, but this was evidence it may be possible.”
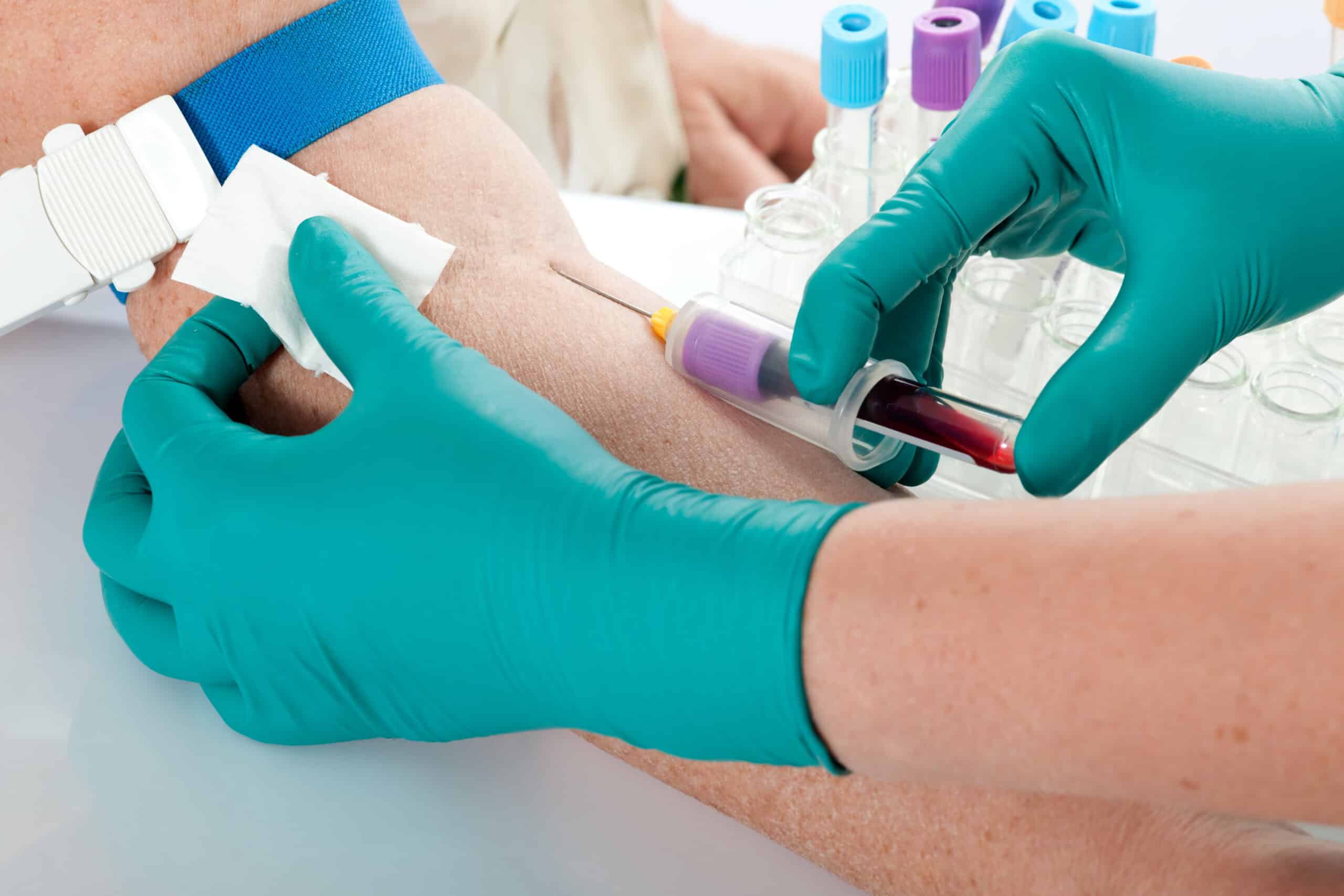
Ilumina created Grail in 2016 in a quest to detect multiple types of cancer before symptoms developing using a single simple blood test, which looks at cell free plasma to find fragments of circulating tumour DNA sloughed off by cancer cells. Early detections means earlier interventions and people may be less likely to die. Most varieties of cancer can only be detected after symptoms appear. A blood test is minimally invasive, simple, and may improve and/or replace other screening programmes with time.
Thanks to advancements in DNA sequencing looking for ctDNA is now a viable proposition to scan for fragments and find those with few alterations which may indicate cancer while other blood based biomarkers are being examined. Advantage of ctDNA is the direct link to the tumour that can be specific at identifying cancer, which is now showing great promise as a way to profile and monitor advanced stage cancers as a liquid biopsy.
The goal is to develop an inexpensive test which can be given annually to those at a certain age or at risk, with a high chance of accurately detecting many cancers at once. “Big clinical studies still need to be conducted, but there has been considerable progress,” says Nitzan Rosenfeld of the Cancer Research UK Cambridge Institute.
Essentially beginning when a normal cell’s DNA gets mutated or altered cancer can move quickly from that point; the cell multiplies too often and a mass/tumour of abnormal cells form. Proportions of cells invariably die and shed genetic material into the bloodstream, which then mix with larger amounts of DNA fragments from the death of normal cells. Fragments of DNA carrying cancer causing mutations being found floating in the blood of cancer patients was first reported in the 90s. Which set in motion research on how to apply ctDNA fragments in the monitoring and detection of cancer.
Guardant Health is also developing a liquid biopsy; as well as John Hopkins University developing CancerSEEK. Queensland researchers have developed Methylscape which suggest it can identify presence of cancer in 10 minutes using gold nanoparticles to detect epigenetic changes. Each are developing their own way of detecting ctDNA, all of which have published or presented results for their methods having demonstrating cancer related signal being seen based on small studies with cancer at various stages.
Key to such tests is achieving high sensitivity and low false positive rates. Grail rates have ranged from 80%-47% for nine cancers with a false positive rate of 2% with further work suggesting it could go lower than 1%. CancerSEEK sensitivity ranged from 98% to 33% with a false positive rate of less than 1%. Guardant showed it can detect lung cancer in 71% of cases and lung cancer in 67% with a false negative rate of 2%. Methylscape had a sensitivity of 90% with a false negative ranging from 10-15%.
While the companies are excited over what they are seeing, not everyone is convinced such as Eleftherios Diamandis who has made a name for himself by criticising “grand designs of blood tests”. Before the media questioned Therano’s blood testing technology Diamandis raised doubts in the scientific literature, the company was charged with massive fraud in 2018.
Diamandis and Clare Fiala have published a series questioning how useful ctDNA testing could be in early diagnosis of cancer. Their calculations are based on experimental literature data to give a sense of tumor size a ctDNA based blood test may be able to pick up, and suggests tumour size would need to be at least 1cm+ in diameter to be detected. “..unlikely to be a single fragment of DNA from tumours under that size in 10ml of blood…..” Diamandis goes on to add the tests are performing well as they as being applied to those already diagnosed with cancer.
Those developing ctDNA blood testing acknowledge that larger studies are needed involving participants that are not diagnosed with cancer. Grail has two large long term studies in the pipeline; and CancerSEEK is looking to enroll 10,000 women not diagnosed with cancer to follow over 5 years.
The question remains as to when we can expect a once a year blood test that is capable of checking for multiple cancers. Experts suggest that it will be at least 5+ years before there is sufficient data to show whether they work, then it will need to pass over the desks of regulators and health economists.
Which brings about breathomics and cancer detection. UK based Owlstone Medical is among those looking to discover whether this is possible and has developed a breathalyser based on the idea of there possibly being chemicals and volatile organic compounds in breath indicative of early stage cancer. The company is investigating whether there are differences between breath from those with and without lung cancer; and has started a smaller study looking at whether 6 other cancer types may also be detected this way. Depending on trial results this may be a more generic cancer detection test that may suggest presence very early on, likely before circulating tumour DNA. Max Allsworth says “..air we breathe is a good place to start as it directly moves through the lungs and past any tumours..”