Specifically, the investigators have improved their understanding of the steps that lead to the production of IL-1 beta, a potent inflammatory protein signal released during many inflammatory responses.
“We now have a clearer understanding of the stepwise process that leads to the production of IL-1 beta,” said Andrea Wolf, Ph.D., assistant professor of Biomedical Sciences and Medicine at Cedars-Sinai, and a senior and corresponding author on the new study. “By understanding the process, we hope to one day find a treatment for diseases associated with this inflammatory response.”
When the innate immune system — the defense system we were born with — identifies a potentially harmful bacterium, virus, or other external invaders, it unleashes white blood cells to surround and attack the foreign agent. This can cause swelling, redness, heat, and pain in the body’s tissues that — in a healthy body — eventually go away.
Some people, however, get stuck in the inflammation phase. This causes what is known as chronic inflammation. Chronic inflammation can damage healthy cells in the body and is thought to lead to serious conditions like Type 2 diabetes, heart disease, and depression.
“Inflammation, in many instances, is vital to a thriving immune system and healthy body,” said David Underhill, Ph.D., chair of the Department of Biomedical Sciences and the Janis and William Wetsman Family Chair in Inflammatory Bowel Disease, who is also a senior and corresponding author on the study. “However, prolonged inflammation can wreak havoc on the body. This underscores the importance of understanding the cellular process of how inflammation is activated so we can work toward finding new treatments to curb chronic inflammation.”
The study published today is a follow-up to Cedars-Sinai’s research published in 2016 that explains how cells act to detect an infection. In that study, investigators discovered that an enzyme called hexokinase, typically used by cells to convert glucose into energy, has a second, inflammatory function. They discovered that hexokinase binds to a sugar from the cell wall of bacteria and activates the inflammasomes, leading to the production of IL-1 beta. Inflammasomes are receptors of the innate immune system that recognize microbes and tissue damage.
The current work presents a more complete picture of this process.
The investigators discovered that hexokinase leaves the mitochondria, the part of a cell that generates energy. This jump-starts an immune response: The release of hexokinase destabilizes the mitochondria and alerts the cell that something is wrong. This leads to the clustering of a channel called VDAC in the membrane of the mitochondria, which interacts with another protein called NLRP3 to initiate inflammasome assembly. The inflammasomes then produce IL-1 beta, a driver of inflammation.
Investigators studied cells that were derived from laboratory mice to understand the steps involved in the IL-1 beta pathway. The team used substances called inhibitors that block cellular functions as well as gene-editing technology to turn off certain genes and the proteins they express. This allowed them to understand which proteins are vital to triggering inflammation.
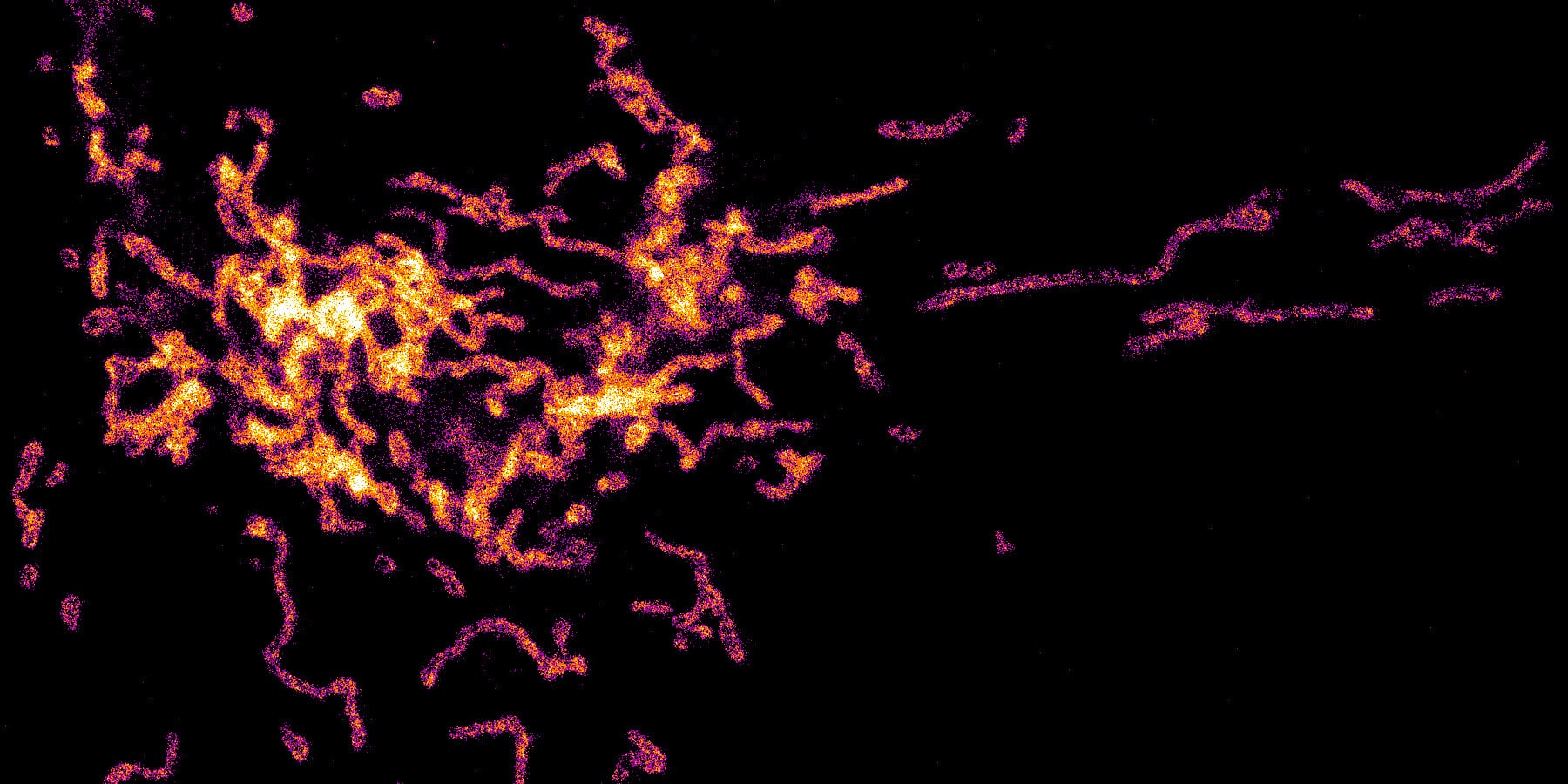
Cedars-Sinai postdoctoral scientist Sung Hoon Baik, Ph.D., used the super-resolution microscope that is part of the Cedars-Sinai Biobank and Research Pathology Resource to visualize and measure the steps of this inflammatory process within individual cells.
“Being able to target specific steps in this pathwayis vital, because in addition to being important for inflammation, the components of this pathway also play a vital role in maintaining energy within the cell,” Wolf said. “We want to home in on its inflammatory role, not just turn it all off, because that would be bad for the cell.”
The investigators are continuing to study the cellular steps leading up to, and resulting from, hexokinase’s role in the activation of inflammasomes. They are also using the results from this study to begin to target this inflammatory pathway in different diseases.
Other Cedars-Sinai investigators who worked on the study include Courtney Becker, manager of the Underhill Laboratory at Cedars-Sinai; Sarah Fett, research associate at Cedars-Sinai; and V. Krishnan Ramanujan, Ph.D., research associate professor in the Department of Medicine at Cedars-Sinai and director of the Cedars-Sinai Biobank.
Funding: The study was funded by the National Institutes of Health (award numbers R01AI148465, R01GM085796, R01AI071116).