Brain cells may play a role in controlling women’s bone density according to research from scientists from UC San Francisco and UCLA which showed blocking particular sets of signals from these cells caused female mice to build extremely strong bones and maintained them into old age; these effects were not observed in male mice.
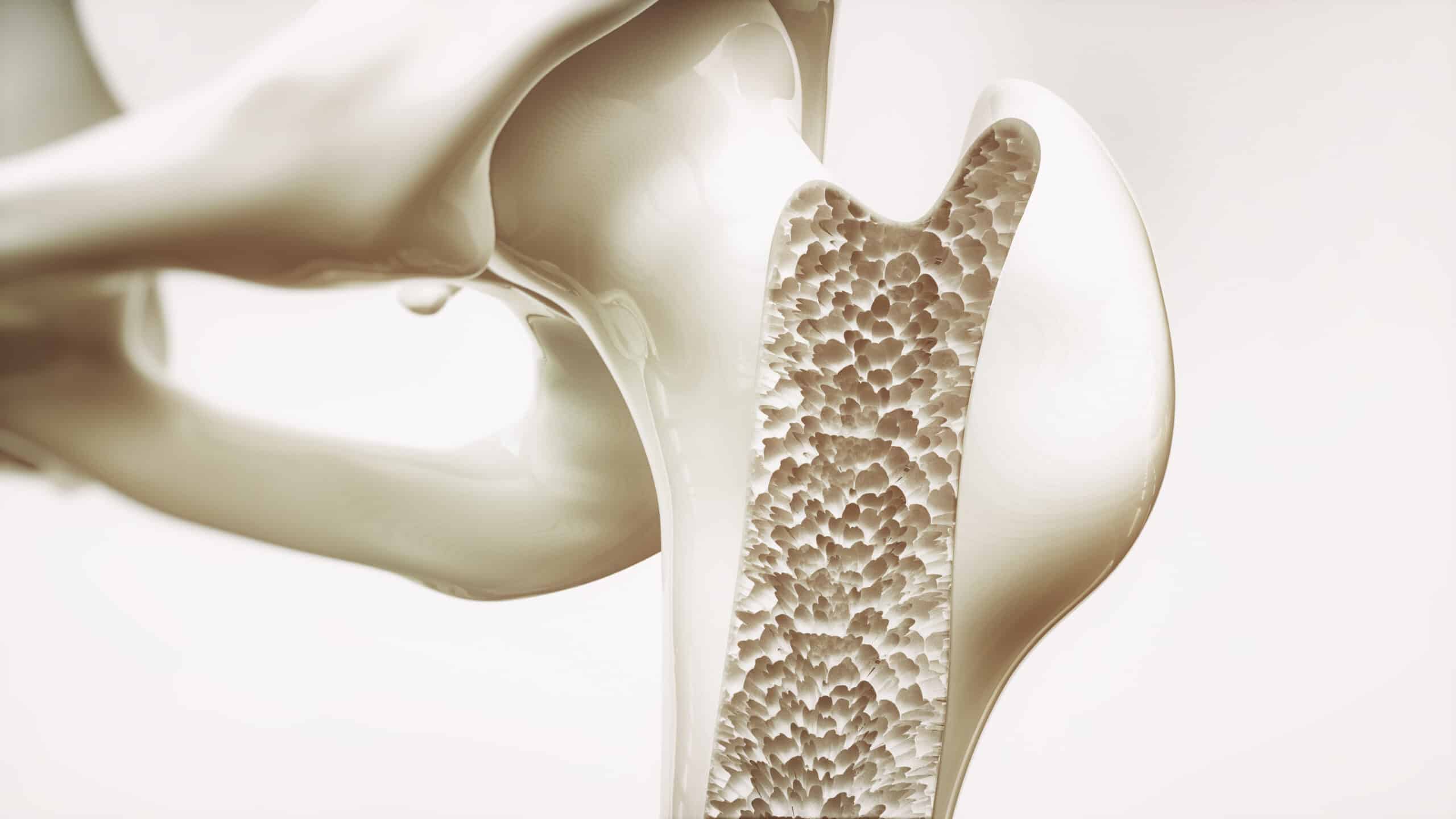
Osteoporosis is the weakening of bones to the point where falls and minor stresses such as bending or coughing can trigger fractures which affects upwards of 200 million people around the globe. Bone tissue is constantly recycled among healthy individuals with old bone tissue being broken down and replaced by new bone, however with age this cycle tilts in favor of bone loss causing bones to become porous and fragile.
After menopause women are at high risk of osteoporosis due to declining levels of estrogen which normally promotes bone growth. Genetically deleting estrogen receptor proteins in hypothalamic neurons was found to have caused mutant animals to gain weight and become less active. To find the source of weight gain a laboratory technique was used which also revealed changes in bone density, which revealed that the mice were truly just big boned as the animals had bone mass increases of as much as 800%.
The extra dense bones were also proved to be extra strong, when testing the mechanical strength of them by crushing them the equipment almost failed according to Holly Ingraham, Ph.D.
A series of studies zeroed in on a specific population of a few hundred estrogen sensitive brain cells in the arcuate nucleus that appeared to be responsible for the increases in bone density; and it was hypothesized that estrogen must signal these neurons to shift energy away from bone growth and deleting the estrogen receptors reversed the shift. Interfering with arcuate estrogen signalling in male mice appeared to have no effect, highlighting the differences in how sex hormones make male and female brains different.
Mutant strong boned animals were revealed to have maintained their enhanced bone density well into old age with further testing. Female mice typically begin to lose bone mass by 20 weeks of age, the mutant animals maintained elevated bone mass well into their second year of life which is a very old age by mouse standards. Existing bone degeneration in models of osteoporosis was even reversed in female mice that had lost more than 70% of their bone density, deletion of arcuate estrogen receptors caused their bone density to rebound by 50% in a few weeks.
Results show the roles estrogen plays in the blood by promoting bone stability, and in the hypothalamus where it appears to restrain bone formation. The scientists hypothesize after puberty the estrogen system in female brains shifts resources away from bone growth towards things such as reproduction which could contribute to women’s higher risk of weakened bones with age.
Such dramatic enhanced bone growth is unlike anything seen in scientific literature, suggesting that Stephanie Correa’s discovery may have uncovered a novel biological pathway by which the brain regulates bone density. The team is investigating exactly how this brain to bone communication happens and whether drugs can be developed to boost bone strength in post-menopausal women.
If the brain is shown to release a novel circulating factor that triggers enhanced bone growth in future studies, it may reveal paths to developing drugs to counteract osteoporosis, suggest Stephanie Correa, Ph. D.